T. Taniguchi, K-C. Wong, L. Nurdiwijayanto, K. Hatakeyama, K. Awaya, S. Ida, K. Koiumuma, S. Ueda, M. Osada, H. Yokoi, "Reversible hydrogenation and irreversible epoxidation induced by graphene oxide electrolysis", Carbon, 177, 26-34 (2021).
Abstract
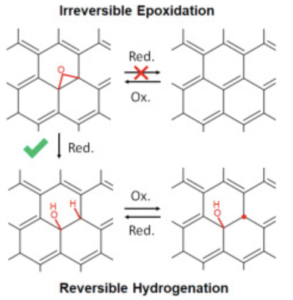
Electrochemical (EC) reduction of graphene oxide (GO) serves as a green chemistry pathway to produce reduced GO (rGO). However, the mechanistic understanding of this process is still limited due to the structural complexity of GO-based materials. In the present study, we employ an inductive approach that consists of investigating the structures afforded by alternating EC reduction and oxidation steps to elucidate the electrochemical reduction reactions in aqueous solution. The initial EC reduction of chemically derived GO mainly converts epoxides (C–O–C) to COH/CH pairs. Simultaneously, carbon-centered radicals (C•) are eliminated. Subsequent EC oxidation does not result in restoration of epoxides but reverse dehydrogenationand radical formation. The second EC reduction and oxidation cycle involves reversible C–H formation coupled with radical elimination, suggesting that C• serves as an active site for the hydrogenation. As a result of this study, COH–CH is revealed as a plausible characteristic structure in electrochemically derived rGO. The reversible radical hydrogenation reaction can offer an effective route to manipulate the chemical reactivities of GO-based materials. We demonstrate that the electrons transferred by EC reduction can delocalize in the rGO to some extent, activating the pseudocapacitance that probably originates from COH.
