Understanding the fast formation mechanism of hard nanoporous alumina films on aluminum in acidic solutions containing nitric acidMater. Chem. Phys. 309, 128271 (2023).
https://doi.org/10.1016/j.matchemphys.2023.128271
Abstract
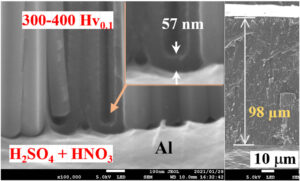
Nanoporous alumina films formed on aluminum via anodization are widely used for scientific and technical purposes owing to their various advantageous properties, including excellent corrosion and wear resistance, good thermal and electrical insulation, and facile producibility at low cost. Herein, we report a novel approach to fabricate hard porous anodic alumina (PAA) films by adding an appropriate amount of nitric acid to sulfuric-, oxalic-, and phosphoric-acid solutions at critical high current densities. The effects of the nitric additives on the anodizing behavior, film growth mechanism, microstructure, chemical composition, film thickness, microhardness, and appearance of the PAA films formed from various solutions were systematically investigated. The addition of nitric acid enhanced the formation of alumina and resulted in fast growth rates of 196 μm/h and 166 μm/h for sulfuric- and oxalic-based solutions, respectively, while maintaining high hardness of approximately 380 and 430 HV0.1, respectively. The surface hardness of the films decreased to some extent with the increase in nitric acid concentration and/or film thickness owing to the strong corrosive nature of nitric acid during prolonged anodization, although nitrogen inclusion in anodic alumina films was not detected through X-ray photo-electron spectroscopy, which differs from the other acidic anions. In particular, the barrier layer thickness and pore intervals of PAA films increased with the nitric concentration in the aforementioned solutions, leading to a fast growth rate and high hardness inside the films.
