Fei Li, Kanako Yoshida, Nguyen Van Chuc, Minoru Osada, Hiroya Abe
Abstract
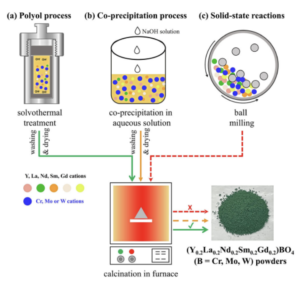
We performed the comparative studies for the synthesis of high-entropy rare earth (Y0.2 La0.2 Nd0.2 Sm0.2 Gd0.2 )BO4 (B = Cr, Mo, W) oxide powders using the polyol process, co-precipitation process, and solid-state reactions. Zircon-type RECrO4 powders and scheelite-type REMoO4 and REWO4 powders were obtained through the polyol process after being post heated at 600 °C and 800 °C, respectively. The powders synthesized from the co-precipitation process and solid-state reactions were not single-phase high entropy oxides, except that the scheelite-type REWO4 powders were obtained through the co-precipitation process. This work demonstrated that the polyol process outperforms co-precipitation and solid-state reaction methods in synthesizing single-phase high-entropy rare earth compounds with group 6 elements.
