Takahiro Kozawa, Tai Hashida, Kayo Fukuyama, Hiroya Abe, Shu Morita, Minoru Osada, Makio Naito
Abstract
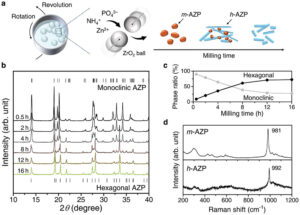
Enhancing NH3 as a carbon-free energy carrier of H2 and next-generation fuel is a promising approach for a sustainable society. Chemically storing NH3 molecules in crystal structures offers better selectivity and reusability than storage in traditional porous materials based on physicochemical adsorption; however, designing materials that can be reversibly stored in structural gaps is still a significant challenge. Herein, the use of NH4ZnPO4, which is previously used as a fertilizer, is proposed as an NH3 uptake material through a chemical storage mechanism. The NH4ZnPO4 particles synthesized by a wet mechanochemical method with monoclinic and hexagonal crystal structures can incorporate NH3 molecules and directly transform them into NH4Zn(NH3)PO4 without producing byproducts. The chemical storage mechanism depends on the particle morphology; therefore, the uptake amount per surface area surpasses that of porous materials. NH4ZnPO4 exhibited excellent cycling performance due to its reusability, which is regenerated by releasing NH3 from NH4Zn(NH3)PO4 when heated in air at ≈100 °C. Taking inspiration from previously used and familiar fertilizers further extends this new area of innovative materials that can be used for the reversible storage of low-molecular-weight gases.
