G. Hasegawa, M. Tanaka, J. J. M. Vequizo, A. Yamakata, H. Hojo, M. Kobayashi, M. Kakihana, M. Inada, H. Akamatsua, K. Hayashi, Nanoscale, 11, 1442–1450 (2019). DOI: 10.1039/C8NR08372J
Abstract
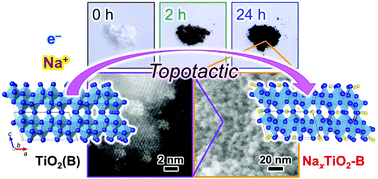
A mixed valence compound, sodium titanium oxide bronze (NaxTiO2-B), combines intriguing properties of high electric conductivity and good chemical stability together with a unique one-dimensional tunnel crystal structure available for cation storage. However, this compound has not been studied for a long period because of the strongly reductive condition at high temperature required for its preparation, which limits the morphological control such as the preparation of nanocrystals. For the first time in this paper, the topotactic synthesis of nano-sized NaxTiO2-B with high specific surface area (>130 m2 g−1) from TiO2(B) nanoparticles has been demonstrated. The reaction of metastable TiO2(B) with NaBH4 allows carrier electrons to be doped simultaneously with incorporation of Na+ ions into the interstitial sites of the host Ti–O lattice at relatively low temperature. An electrochemical investigation of Li+- and Na+-ion storage behaviors suggests that the incorporated Na+ ions are mainly placed in the 6-fold coordination sites of bronze. In addition, optical measurements including time-resolved transient spectroscopy revealed that the doped electrons in the NaxTiO2-B nanoparticles are predominantly in the Ti3+ state and behave as a small polaron. The pelletized NaxTiO2-B nanoparticles shows a good electronic conductivity of 1.4 × 10−2 S cm−i at 30 °C with an activation energy of 0.17 eV, which is attributable to the thermal barrier for the polaron hopping.